Chemical Bonds
When atoms of different elements combine together they form compounds. Familiar compounds include common table salt (Sodium Chloride) and water. Table salt is made from a combination of atoms of sodium (Na) and chlorine (Cl) in a ratio of 1:1 forming the compound NaCl. Water is a combination of hydrogen (H) and oxygen (O) is a ration of 2:1 forming the compound H2O.
There are different types of chemical bonds. Some bonds involve a transfer of electrons. Others involve a sharing of electrons. Still other bonds are weak attractions between molecules. Let's look at each type of bond.
1. Ionic Bonds.
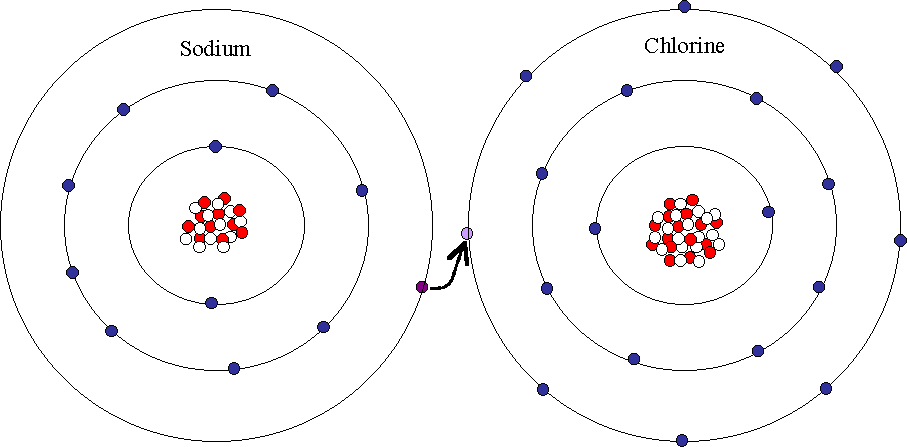
Ions are formed by atoms that have non-full outermost electron shells in order to become more like the noble gases in Group 8 of the Periodic Table (see section on ions). Some atoms add electrons to get a full shell, thus becoming a negative ion. Other atoms subtract electrons from their outermost shell, leaving a full shell and an overall positive charge on the ion. In the previous section, we saw that atoms with fewer than 4 electrons in their outermost shell tend to form positive ions, and those with more than 4 electrons tend to form negative ions. Ionic bonds form when atoms transfer electrons between each other, forming ions that are electrically attracted to each other forming a bond between them. Sodium chloride (NaCl) is a typical ionic compound. The picture below shows both a sodium and a chlorine ion.

Sodium has 1 electron in its outermost shell, and chlorine has 7 electrons. It is easiest for sodium to lose its electron and form a +1 ion, and for chlorine to gain an electron, forming a -1 ion. If sodium can transfer it's "spare" electron to chlorine (as shown above), both atoms will satisfy their full outer shell requirements, and an ionic bond will be formed. If large groups of sodium and chlorine atoms bond this way, the result is a three-dimensional structure with alternating sodium and chlorine ions:
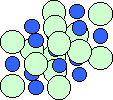
The blue dots are the sodium atoms; the pale green dots are the larger chlorine atoms. Ionic bonds between each atom forms a relatively strong bond and a three-dimensional, cubic structure. Below is a look at just a single layer:
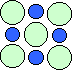
Note that each positive sodium ion is next to a negative chlorine ion. Now imagine this arrangement continuing outward in all directions with thousands of billions of atoms. Wow!
2. Covalent Bonds.
Sometimes atoms will share electrons instead of transferring them between the two atoms. This sharing allows both atoms to fill their outermost shell while forming a very strong bond between the atoms. Elements such as carbon (C) and Silicon (Si) form strong covalent bonds. Below is a picture showing the electron sharing that occurs in the mineral diamond. Diamonds are made of pure carbon and its the way that the carbon atoms are bonded that makes diamond the hardest substance.
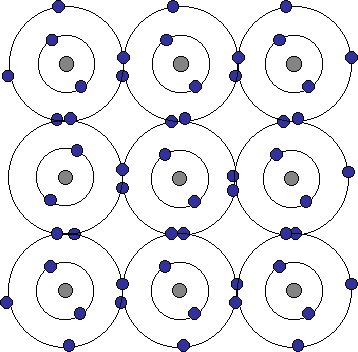
Each carbon atom has 4 electrons (blue dots) in its outer shell. This allows the atom to share electrons with 4 other carbon atoms surrounding it (as the middle carbon atom is doing). Each of these in turn will share the remaining 3 electrons with adjacent carbon atoms beside, above and below it, and those with other carbon atoms, etc., forming a interlocking, three-dimensional network of tightly bonded carbon atoms. Similarly, covalent bonding between silicon and oxygen atoms makes strong bonds that form a large group of minerals called silicates (more on those later).
3. Metallic and Van der Waals Bonds.
Metallic bonds form when the outer shell electrons are shared between neighboring atoms. Unlike covalent bonding however, there are insufficient numbers of electrons in most metal atoms (such as copper or silver) to form pure covalent bonds. Therefore, the electrons are shared amongst all the nearest neighbor metal ions, forming a metallic bond. This strange arrangement of "metallic ions is a sea of electrons" gives metals their particular physical properties.
Metallic bonds are also explained by band theory. Band theory states that closely packed atoms have overlapping electron energy levels resulting in a conduction "band" wherein the electrons are free to roam between atoms, thus bonding them together. For more information on metallic bonds and band theory, see this web site.
Van der Waals bonds are weak bonds that form due to the attraction of the positive nuclei and negative electron clouds of closely packed atoms. This attraction is opposed by the repulsive force of the electron clouds and the repulsive force of neighboring nuclei. However, the attraction is stronger than the total repulsive forces, leaving a residual, weak attraction. Van der Waals bonding is important in minerals such as graphite and clay minerals.
Test your knowledge with a quiz!
Return to main menu.
Return to Introductory Geosciences Course Page.