Ions
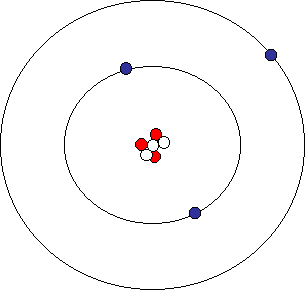
Look at the atom shown below. It has 3 each of protons, neutrons and electrons, and represents that element Lithium (Li). If we were to write out the name symbolically, it would be 6Li.

Lithium Atom
Lithium has only one electron in it's outermost shell. What would happen if we were to remove that electron?
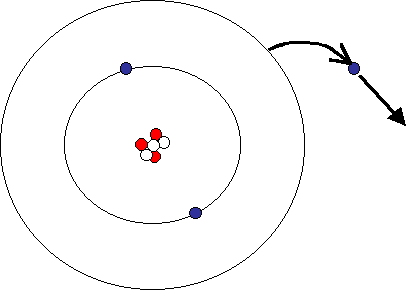
Without its outermost electron, the lithium atom would have more positive charges (+3) than negative charges (-2). An atom with a different number of electrons to protons would be called an ion. Elements like lithium that loose their electrons form positive ions. Symbolically, we can represent this as Li+1. Other elements tend to gain electrons. Oxygen is a good example of one of these:
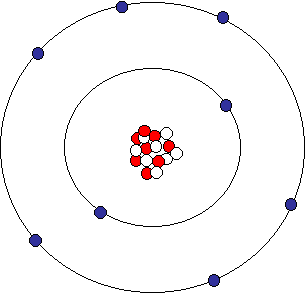
Oxygen Atom
Oxygen has a total of 8 electrons normally, but only 6 of these are in the outermost shell or orbital. Elements prefer to have full outer shells. They also prefer to get to this state as easily as possible. Above, it was easier for lithium to lose one electron than to gain 7 electrons. Similarly, it is easier for oxygen to gain 2 electrons instead of loosing 6 electrons:
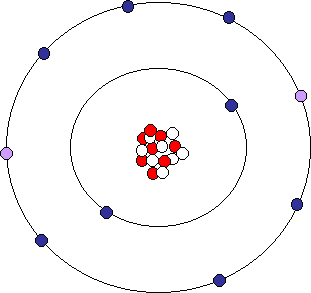
Oxygen Ion
The two gained electrons (purple dots) means that this oxygen ion has 10 electrons (-10 charge) and only 8 protons (+8 charge), giving the ion a net charge of -2. Symbolically, we can represent this oxygen ion as O-2.
The Periodic Table can be used to help predict how many electrons there are in the outermost shell, and hence what type of ion they will form. Here is the same chart from the previous page, but with some additional information added to it:

The black numbers above represent the number of electrons in the outer shell. Notice that each column has more electrons in the outermost shell as you go to the right, and that the last row (headed by He or Helium) has a full outer shell. {Special note: Helium has only 2 electrons in the outermost shell, but that is full for the first shell.} The red numbers represent the type of ion that the atom would form, starting with +1 ions on the left and finishing with no ions ("0") on the right. Elements with a full outer shell do not form ions. The yellow section, labeled "Transition Elements" are elements that tend to lose electrons from shells other than the outermost shell and form positive ions. For example, iron (Fe) forms two different positive ions, Fe+2 (ferrous iron) and Fe+3 (ferric iron). Understanding why there are different ions of iron is complex and beyond the scope of this course. However, you should be able to determine the ionic state of atoms from the other groups using a Periodic Table.
Quick Quiz: Use the chart above to answer the following questions:
What ion would a Chlorine (Cl) atom form?
What ion would an Aluminum (Al) atom form?
What ion would a Magnesium (Mg) atom form?
What ion would a Potassium (K) atom form?
Answers:
Chorine is in the seventh column and therefore has 7 electrons in its outermost shell. It would tend to gain one electron and form a -1 ion.
Aluminum is in the fifth column and therefore has 5 electrons in its outermost shell. It would tend to lose three electrons and form a +3 ion.
Magnesium is in the second column and therefore has 2 electrons in its outermost shell. It would tend to lose two electrons and form a +2 ion.
Potassium is in the first column and therefore has 1 electron in its outermost shell. It would tend to lose one electron and form a +1 ion.
Continue and learn about bonding.
Return to main menu.
Return to Introductory Geosciences Course Page.