 Igneous and Metamorphic
Petrology
Igneous and Metamorphic
Petrology
GEOL-4711 Spring 2014
Review for 1st Lecture Test
Fundamental Concepts (Chapter 1)
1) What is the definition of petrology? What is the difference between petrology and petrography, petrochemistry and petrogenesis?
2) Describe the thickness, average density and composition of earth’s crust, mantle and core. Describe the origin of the crust.
3) Compare and contrast oceanic and continental crust. In this discussion include a description of differences in composition, age, thickness, origin and distribution.
4) Describe the general characteristics of the mantle. How is this layer distinguished from the overlying crust?
5) List and describe the three major compositional layers of the mantle. What are the transitional zones? How are they recognized? Describe the proposed phase changes that cause these changes.
6) List and describe the major tectonic layers of the mantle. How are these recognized? Why are they important?
7) Describe the two major components or layers of the core. Describe how these areas are recognized. Why are they important?
8) Describe the three major types of meteorites. Which of these are most abundant? Explain why meteorites are believed to be pieces of ‘failed’ planets? Compare and contrast chondrites and achondrites. Why are chondrites used to estimate the composition of the original solar nebula and primitive inner planets?
9) List the 7 most abundant elements that make up more than 97% of the earth. Next, list the 8 most abundant elements in the crust. Explain why these are different. What is the Oddo-Harkin Rule?
10) Describe the proposed origin of earth’s compositional layers.
11) Describe the two major sources of heat within the earth. What is the geothermal gradient? Why does this gradient change with depth? How is heat transferred within the earth? Give examples of conduction, convection and advection within the earth.
12) What is lithostatic pressure? Write the equation that is used to determine the lithostatic pressure at depth. Be able to calculate the pressure at different depths.
13) What is adiabatic heating and compression? Explain why adiabatic heating is probably not very important in magma production.
14) Define the following terms: lherzolite, spinel, perovskite, low velocity zone, mesosphere, asthenosphere, lithosphere, Dynamo Theory, advection, and convection.
Description and Classification of Igneous Rocks (Chapter 2)
1) Compare and contrast the following: phaneritic, aphanitic and porphyritic. How do each of these textures form? Describe two ways by which fragmental textures form. How can each origin or method of formation be distinguished in hand sample and thin section?
2) Describe how cooling rate determines crystal nucleation and growth rates. Why does slow cooling result in larger crystals of limited phases?
3) List and describe the factors that control crystal form. Define the following: euhedral, subhedral and anhedral. Describe how the sequence of crystallization can usually be determined by crystal form.
4) Define and draw simple sketches of the following textures: cumulate, pilotaxitic, vesicular, and zoning. Compare and contrast normal, reverse and oscillatory zoning.
5) Describe how pyroclastic rocks are formed and how they are recognized in hand sample and thin section.
6) Compare and contrast primary and secondary textures in igneous rocks. Give examples of each. Describe how the following post-solidification textures form and give mineral examples of each: polymorphic transformations, exsolution, transformation twins, and devitrification. Name and describe two major types of alteration textures.
7) Define, compare and contrast modal and normative mineral compositions. Why are most major modal mineral phases in igneous rocks some combination of the minerals found in Bowen’s Reaction Series plus Fe-Ti oxides? What are accessory minerals?
8) Describe the basis for the chemical classification of igneous rocks by silica content. Name examples and give the silica content of the following compositions: silicic, intermediate, mafic and ultramafic. How are silica saturated, oversaturated and undersaturated rocks recognized?
9) Define and describe peraluminous and peralkaline igneous rocks.
10) What is the basis for the IUGS Classification of Igneous Rocks? Why are different diagrams used for different geochemical rock groups? Be able to plot rock composition data on IUGS Classification diagrams.
11) Define the following terms: autobrecciation, lapilli, eutaxitic, fiamme, pegmatite, and graphic intergrowths.
Thermodynamics (Chapters 5 and 6)
1) Define thermodynamics. What is the scientific basis for the proposed phase reactions and stability ranges?
2) Define and describe equilibrium. Why are many minerals and igneous systems considered metastable? Compare and contrast systems and surroundings.
3) Compare and contrast intensive and extensive state properties. Give examples of each. Define and describe the following extensive properties: internal energy, entropy and enthalpy.
4) Define GIibbs Free Energy and describe how it is used to predict the direction of change in a system. Write the two most common formulas used in determining the Gibbs Free Energy of a system.
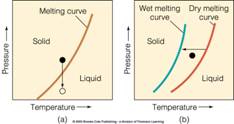
5) What are phase diagrams and how are they constructed? Describe and give examples of one, two, and three component phase diagrams. Write and describe the Phase Rule.
6) What is the Lever Rule? Be able to use the Lever Rule to determine the abundance of phases in both binary and ternary phase diagrams.
7) Define and describe each of the following types of melt-crystal interactions: equilibrium crystallization, fractional crystallization, equilibrium melting, fractional melting and partial melting. Be able to recognize and draw each type of interaction on both binary and ternary phase diagrams.
8) Be able to give mineral examples and draw phase diagrams for each of the following: binary solid solution, binary eutectic, binary exsolution, and binary peritectic systems.
9) Describe and draw the Di-An-Fo Ternary System. Be able to plot compositions on the diagrams and predict melting or crystallization histories. Why is this system so important?
 10) Define the following terms: activation
energy, liquidus, solidus, solvus, invariant point, and eutectic point.
10) Define the following terms: activation
energy, liquidus, solidus, solvus, invariant point, and eutectic point.