Atomic Structure
All matter is formed from basic building blocks called atoms. Atoms are made of even smaller particles called protons, electrons, and neutrons. Protons and neutrons live in the nucleus of an atom and are almost identical in mass. However, protons have positive charges whereas neutrons have no charge. Electrons have a negative charge and orbit the nucleus in shells or electron orbitals and are much less massive than the other particles. Since electrons are 1836 times less massive than either protons or neutrons, most of the mass of an atom is in the nucleus, which is only 1/100,000th the size of an entire atom(!).
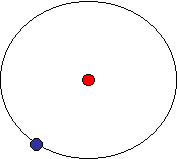
The organization of a hydrogen atom is as shown below:

Hydrogen atom
The red dot is a proton in the nucleus. It has a positive charge of +1 unit. The blue dot is an electron. It has a negative charge of -1 unit. For any normal atom, the number of electrons and protons is equal, meaning the electrical charge is balanced. There is only one orbital for hydrogen. Let's look at a larger atom, carbon.
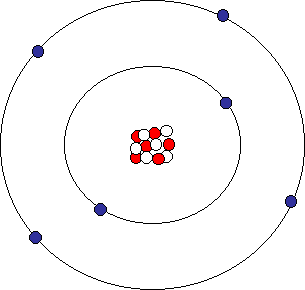
Carbon atom
There is now a new particle in the nucleus, the neutron (represented by the white dots). There are also 6 protons in the nucleus, for a total of 12 particles. In addition, there are now 6 electrons orbiting the nucleus in two orbitals. The reason the carbon atom needs a second orbital are complex and are beyond the scope of this geology class. But the rules that govern atoms say that the first orbital can only have two electrons, the second orbital is allowed eight electrons, the third orbital only eight electrons, etc. (See page 27 of your text to the numbers of electrons in each orbital for the first 20 elements.)
There are 91 naturally occurring elements. Atoms are the smallest pieces of an elements possible, and in fact the word "atom" comes from the Greek word "a tomos" which means "not cutting" -- i.e. you can't cut it any smaller than that. We usually represent elements by their atomic symbol. Hydrogen is represented by an "H"; carbon by a "C".
For atoms, changing the number of protons changes the kind of element. So, if I were to drop an additional proton into the nucleus of the carbon atom illustrated above, I would no longer have carbon -- I would have nitrogen. Similarly, if I took away a proton from the carbon atom, I would have another element, boron. The number of protons in the nucleus of an atom is the same as the atomic number of that atom. If you add together the number of protons and neutrons, you get the atomic mass number of that particular atom.
Quick quiz: What is the atomic number of the hydrogen atom shown above? What is it's atomic mass number? What is the atomic number and atomic mass number of the carbon atom shown above?
Answers: The atomic number of hydrogen is 1 (count the protons). The atomic mass number of hydrogen is also one (there are no neutrons!). For carbon, the atomic number is 6, and the atomic mass number is 12 (6 protons plus 6 neutrons).
Look at the illustration of the carbon atom again. What if we added a neutron instead of a proton? Would we have the same element? Yes. But, the atom would be different. Adding or subtracting neutrons from the nucleus of an atom creates isotopes of that atom. For instance, lets add two neutrons to the carbon atom, represented by green dots below:
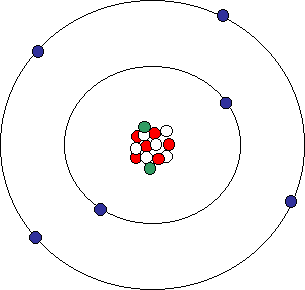
Carbon isotope
Adding the two neutrons changes our atom. However, because the number of protons are the same, it is still carbon but now it is an isotope of carbon. We represent isotopes by using the chemical symbol ("C" for carbon) and a number. The first carbon atom with only 6 neutrons would be called 12C or Carbon-12. The new one with 8 neutrons would be 14C or Carbon-14. Note that the number "14" is also the atomic mass number for this isotope.
Chemists have worked to organize the elements in a particular way called the Periodic Table. It is ordered such that elements in each column have certain chemical and physical properties in common. Below is an image of the Periodic Table:
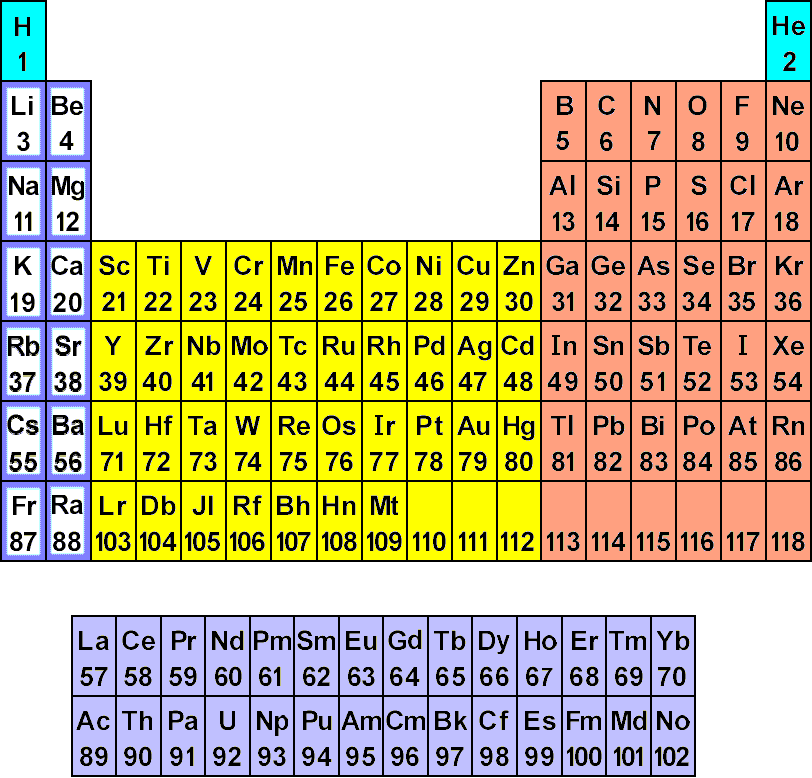
*Image from http://www.chemtutor.com/perich.htm
Each element has an atomic symbol and an atomic number.
Quick Quiz: Recall that the atomic number is the number of protons in the nucleus. What is the number of protons in a Sodium (Na) atom? An Oxygen (O) atom? A Uranium (U) atom?
Answers: Sodium has 11 protons, Oxygen 8
protons, and Uranium 92 protons.
Continue and learn about ions.
Return to main menu.
Return to Introductory Geosciences Course Page.
(For more information on basic chemistry and atomic structure, visit http://www.chemtutor.com/.)